ASLAN Pharmaceuticals (ASLN) is a micro-cap biopharmaceutical company focused on developing therapies for the treatment of cancers, autoimmune and inflammatory diseases in the Asia-Pacific region.
ASLAN’s clinical-stage candidates are being developed in multiple therapeutic areas, including oncology, immunology, and gastroenterology. ASLAN is also developing a pipeline of immuno-oncology assets designed to harness the power of the immune system to fight cancer.
ASLAN does not currently have an approved drug in its pipeline, with the TREK-AD and TREK-DX as the most advanced trials in its pipeline. So any upcoming catalyst is binary event for ASLN. Strong cash position ($69M) with expected runway to late 2023.
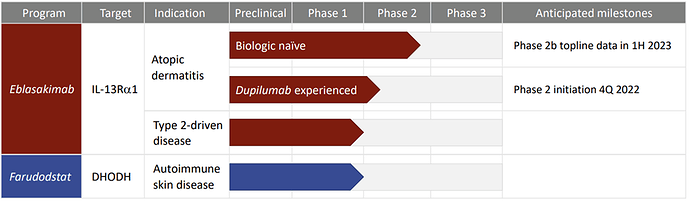
Pipeline (Focus is on Eblasakimab and Atopic Dermatitis (AD))
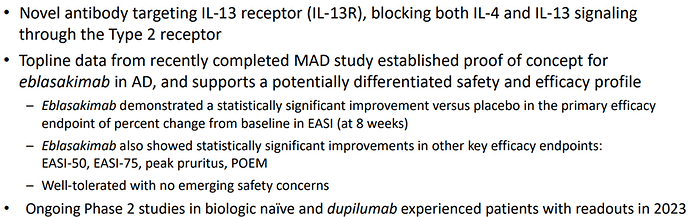
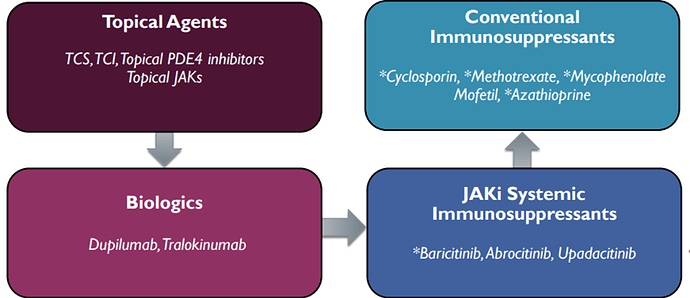
Eblasakimab, is a potential first-in-class antibody targeting the IL-13 receptor that has the potential to improve upon current biologics used to treat allergic disease. Eblasakimab is the only monoclonal antibody in the clinic targeting the IL-13 receptor:
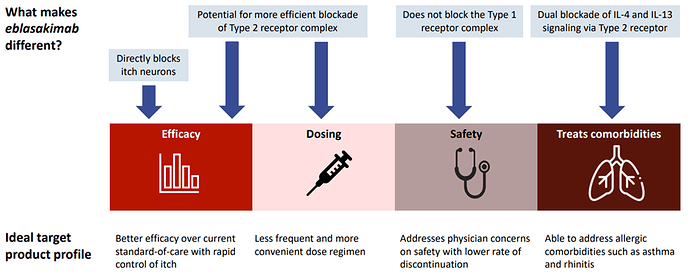
Eblasakimab has the potential to be a differentiated therapy in AD:
Catalysts and progress in the past 12 months:
- Positive topline data readout from Phase 1b MAD study in Sep 2021
- Presentation of results from MAD study: Late Breaker Oral Presentation at AAD 2022 and 3 posters at EADV 2022
- TREK-AD: Phase 2b study initiated
- Data supporting differentiation: novel translational work on neuronal itch, late-breaker at SID 2022, 2 late-breakers accepted at ESDR 2022
- Initiating TREK-DX: pioneering study for dupilumab-experienced patients
Upcoming Catalyst (Q2 2023)
In the second quarter ASLAN will report data from its Phase 2b TREK-AD trial of Eblasakimab (ASLAN004) to treat atopic dermatitis (AD). The primary endpoint of the trial is measured by the percent change from baseline in Eczema Area and Severity Index (EASI) at Week 16.
Study Design:
- The study is expected to enroll approximately 300 adult patients across 100 sites in North America, Europe and Asia Pacific and will consist of a 16-week treatment period and a 12-week safety follow-up period. I see 295 confirmed Participants .
- ASLAN expects to report topline findings from the 16-week treatment period in the first half of 2023.
- The randomized, double-blind, placebo-controlled, dose-ranging clinical study will evaluate the efficacy and safety of eblasakimab in adult patients with moderate-to-severe AD who are candidates for systemic therap
- The TREK-AD study will randomize patients equally to four active treatment arms and one placebo arm, evaluating eblasakimab 300mg dosed every two weeks, 400mg dosed every two weeks, 400mg dosed every four weeks and 600mg dosed every four weeks.
- The primary efficacy endpoint is percentage change in Eczema Area Severity Index (EASI) score from baseline to week 16.
- Key secondary efficacy endpoints include the proportion of patients achieving Investigator Global Assessment (IGA) score of 0 (clear) or 1 (almost clear), proportion of patients with a 75% or greater reduction in EASI (EASI-75), proportion of patients achieving EASI-50 and EASI-90, and changes in peak pruritus.
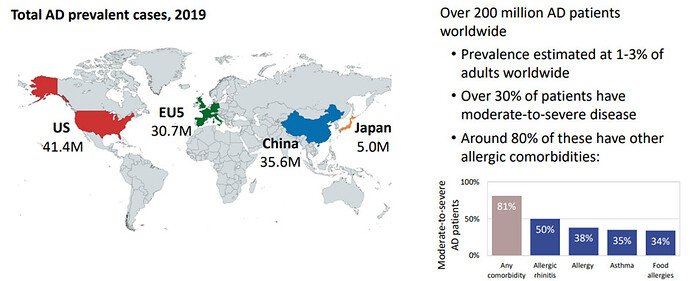
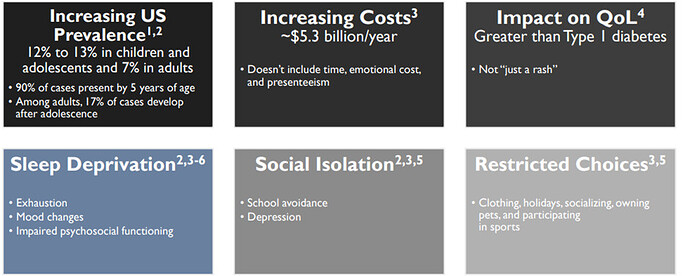
Emerging unmet needs for Atopic Dermatitis (AD)
The total Atopic Dermatitis (AD) Market prediction is $24 billion in 2029. There are two FDA approved drugs to treat atopic dermatitis, dupilumab and tralokinumab:
*not approved in the US for treatment of AD
Dupilumab has advanced the standard of care for atopic dermatitis but a significant unmet need remains:
- There are few safe and effective treatments for moderate-to-severe AD
- Treatment is traditionally focused on topical corticosteroids but steroid use can be associated with safety risks
- Dupilumab has established dual blockade of IL-4/IL-13 biologic therapy as the new standard of care1. Launch of dupilumab in 2017 helped drive a large market for systemic AD therapy with 2021 sales of $5.2. Sanofi expects to grow sales to over $14B
However, only 8% of eligible patients receive dupilumab today and there remains a significant unmet need: Only 35% of patients treated with dupilumab achieved an optimal response, Conjunctivitis is common and can lead to treatment discontinuations Opportunity to improve upon biweekly dosing regimen.
Other Catalyst
- Eblasakimab TREK-AD topline data expected 2Q 2023
- Eblasakimab TREK-DX topline data expected 1Q 2024
Position: will start a position on Monday.