Vaccinex (VCNX) is pioneering a differentiated approach to treating neurodegenerative disease through the inhibition of semaphorin 4D (SEMA4D), a key driver of neuroinflammation. The company’s lead drug candidate, pepinemab, blocks SEMA4D and has potential as a disease-modifying treatment for Huntington’s, Alzheimer’s and other neurodegenerative diseases.
Pepinemab
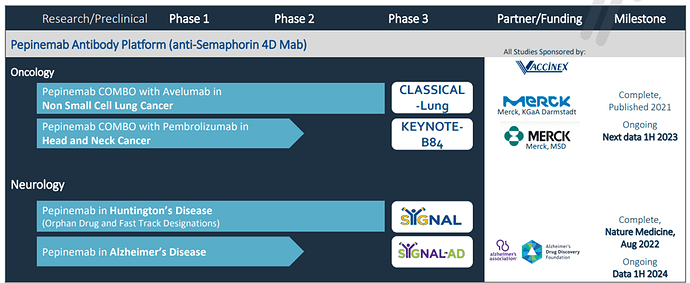
Pepinemab is a humanized IgG4 monoclonal antibody that inhibits SEMA4D, which regulates the actin cytoskeleton of cells that plays an important role in tumor immunity and in inflammatory reactions in the brain. Preclinical and clinical data show that by preventing inflammatory reactivity of pepinemab during disease progression, pepinemab preserves normal function of astrocytes and microglia, two types of glial cells that play a crucial role in the development and maintenance of neurons in the brain. Additional data show that pepinemab promotes infiltration and activation of dendritic cells and CD8+ T-cells and reverses immunosuppression within the tumor microenvironment. Pepinemab is being evaluated in several studies in oncology and neurodegenerative disease.
Pipeline and Milestones:
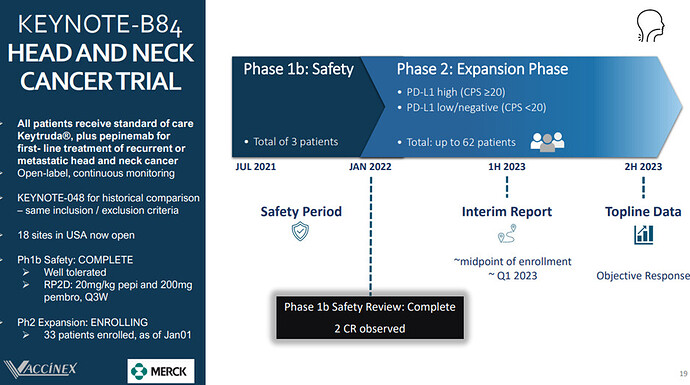
Upcoming Catalyst: In Q1Vaccinex is set to release an interim analysis from its Phase 1/2 KEYNOTE-B84 trial of Pepinemab and KEYTRUDA (pembrolizumab) as a treatment for recurrent or metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC).
I see the opportunity as well for an M&A play given the strong partnership with MERCK.
Position: Shares (announced it in the position system a while ago)