Disclaimer (Position): The company and the upcoming catalyst gets a lot of attention, so I am not sure yet how to play it but started a small shares position.
Achieve Life Sciences (ACHV) is a specialty pharmaceutical company committed to addressing the global smoking health and nicotine addiction epidemic through the development and commercialization of cytisinicline.
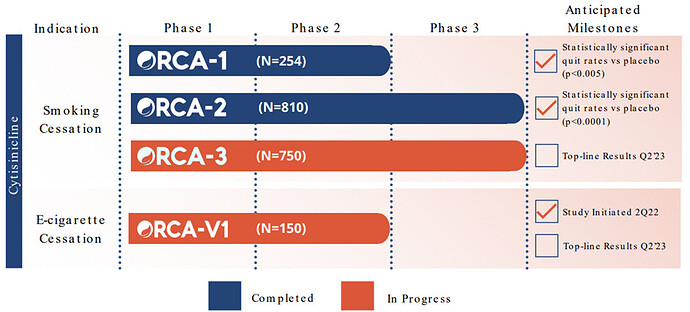
Pipeline
Upcoming Catalyst: Topline results from Cytisinicline (ORCA-3) to treat smoking cessation and nicotine addiction expected in 2Q 2023. This is a binary event as the Phase 3 is seen as registrational trial, so basically it will be the data basis for the submission to the FDA for drug approval.
Previous data shows us the following:
-
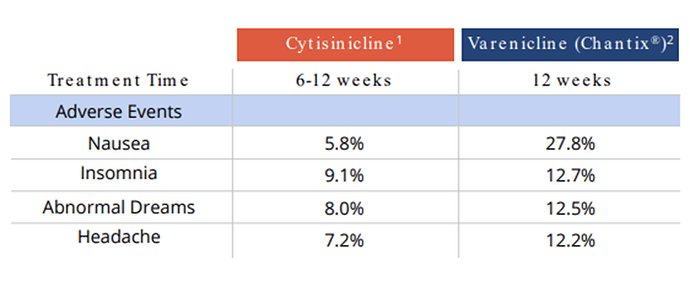
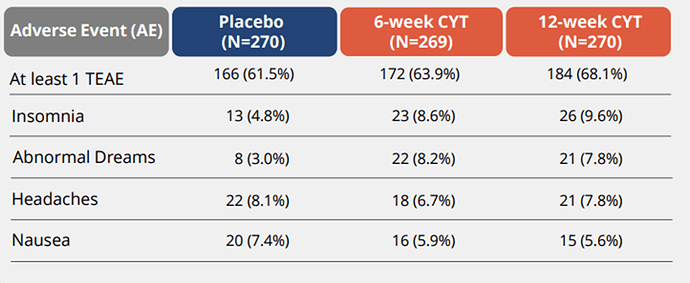
Safe and well-tolerated compared to Chantix and Placebo:
-
Strong Relative Efficacy compared to current treatments:
The ORCA-3 trial mirrors the Phase 3 Orca-2, so following design:
- N=750 (Smokers of ≥10 cigarettes/day and expired air CO > 10 ppm) , 20 sites in the U.S.
- Completed enrollment in September 2022
- Evaluate safety and efficacy of 3.0 mg of cytisinicline vs placebo administered 3x daily (TID) for 6 & 12 weeks
- All subjects received standard behavioral support and were followed out to 24 weeks
- Multiple Primary Endpoitns: Biochemically verified continuous abstinence during the last 4 weeks of treatment (Arm B: Weeks 3-6; Arm C: Weeks 9-12)
- Secondary Endpoint: Continuous abstinence from end of treatment through week 24
Market Opportunity and lack of innovative treatment options
- 1.1b Global Smokers based on a WHO Report on the Global Tobacco Epidemic from 2019
- ~480K Deaths in the U.S. based on Data from U.S. Department of Health and Human Services from 2022
- 8 to 11 estimated attempts before quitting successfully.
- 4 out 5 patients relapse six months post-initiation of treatment with Chantix /Generic version of Chantix (standard of care)
- No new FDA-approved treatments in the last 15 years
- Even with a strong trend of global health campaigns and increased awareness the US tobacco Market is still strong and hence the related disease are still a big burden to the healthcare system.
Financials / IP
- U.S. Patent and Trademark Office (USPTO) issued Patent No. 11,459,328 covering the mesylate salt formulation of cytisinicline and the process for its development.
The recent private placement seems to include warrants as well, ~4.1M units at $4.625/unit, with each unit consisting of two shares and one warrant to buy a share. Therefore, if not already exercised, there is a chance of around 4.1M additional shares (around 20M if fully diluted) that can potentially dilute the current stock price, which is a potential concern down the road.