Disclaimer: This is a lottery ticket play, expect to lose your money.
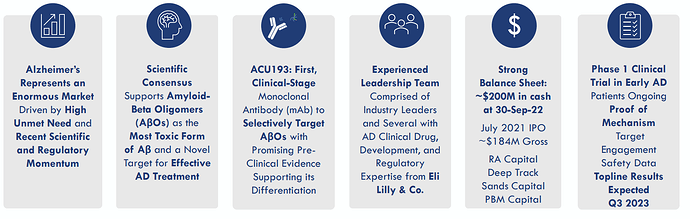
Acumen (ABOS) is a clinical-stage biopharmaceutical company developing a novel therapeutic that targets toxic soluble amyloid beta oligomers (AβOs) for the treatment of Alzheimer’s disease (AD). Acumen’s scientific founders pioneered research on AβOs, which a growing body of evidence indicates are primary triggers of Alzheimer’s disease pathology.
Pipeline
Acumen is currently focused on advancing its investigational product candidate, ACU193, a humanized monoclonal antibody that selectively targets toxic soluble AβOs in INTERCEPT-AD, a Phase 1 clinical trial involving early Alzheimer’s disease patients. The Anti-Amyloid-ß Oligomer Antibody ACU193 Gained FDA Fast Track Designation as well.
Upcoming Catalyst: Phase 1 top-line data due in 3Q 2023
-
INTERCEPT-AD: Phase 1 clinical trial of ACU193 in patients with early Alzheimer’s disease (AD) (RCT)
-
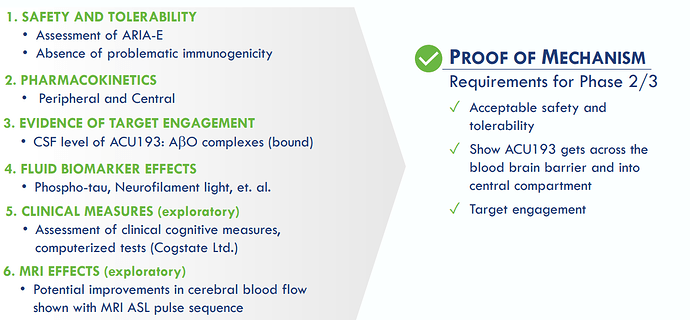
Topline results, safety and clinical proof-of-mechanism following full database lock expected in Q3 2023
-
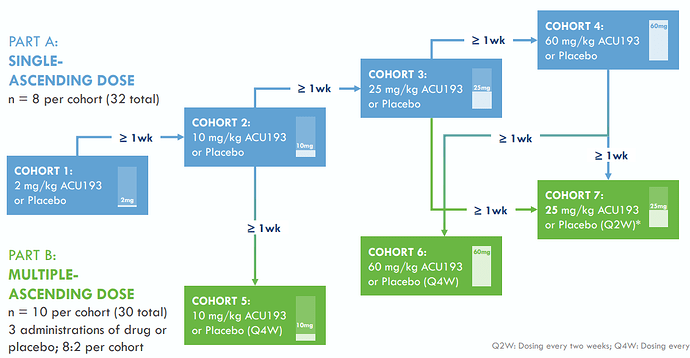
The Phase 1 INTERCEPT-AD trial enrolled 65 subjects across 17 active sites in the United States
-
The INTERCEPT-AD study consists of single-ascending-dose (SAD) and multiple-ascending-dose (MAD) cohorts and is designed to evaluate the safety, tolerability, pharmacokinetics (PK), and target engagement of intravenous doses of ACU193:
-
INTERCEPT-AD a Randomized Placebo Controlled Phase 1 in Early AD patients*:
-
Anticipate next clinical study, with success in Phase 1, starting as Phase 2 study
with potential to expand to Phase 3 registration study based on interim expansion
analysis
*on January 30, 2023, Acumen submitted a protocol amendment to FDA to reduce the dose in Cohort 7 to 25 mg/kg Q2W from 60 mg/kg Q2W. This was based on a blinded review of
preliminary pharmacokinetic data, inclusive of plasma and CSF levels, that indicate a dose of 60 mg/kg Q2W should not be needed to attain central target engagement, and preliminary
safety data, inclusive of two asymptomatic cases of ARIA-E. While ACU193 is early in clinical development, the incidence of ARIA-E to date is consistent with our previous expectations
regarding the safety profile of ACU193. The dose of ACU193 in Cohort 6 (60 mg/kg Q4W) has been maintained as planned.
Extensive Pre-Clinical Data Package supported the continued development:
Financials:
Acumen is Well Capitalized (~$200M) with Expected Cash Runway Through 2025
Position: Some lottery tickets