Disclaimer: This is more up for discussion, no complete DD
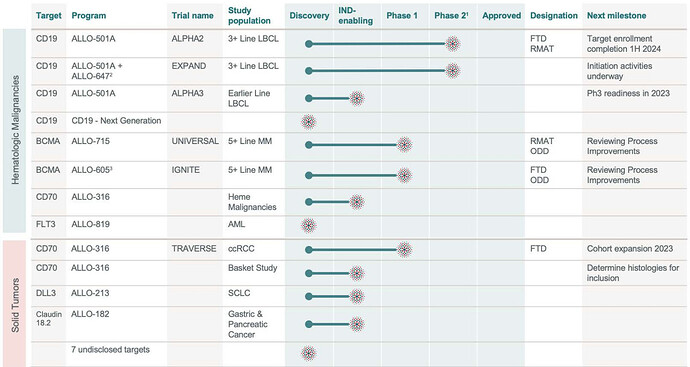
Allogene Therapeutics (ALLO) is a clinical stage immuno-oncology company focused on the development and commercialization of genetically engineered allogeneic T cell therapies for the treatment of cancer. Allogene’s lead product candidates include ALLO-501, ALLO-501A, ALLO-715, ALLO-715 plus nirogacestat, ALLO-605, ALLO-316, and ALLO-647:
Upcoming Catalysts
- Phase 1 ALPHA/ALPHA2 trials of ALLO-501/501A data update to be presented at ASCO 23 (June 3)
ALLO-501 and ALLO-501A
- ALLO-501 and ALLO-501A are anti-CD19 AlloCAR T investigational products for the treatment of large B cell lymphoma.
- Inical Focus is on Large B-cell lymphoma (LBCL) and Non-Hodgkin lymphoma (NHL), the platforms allows for use in a broader patient population
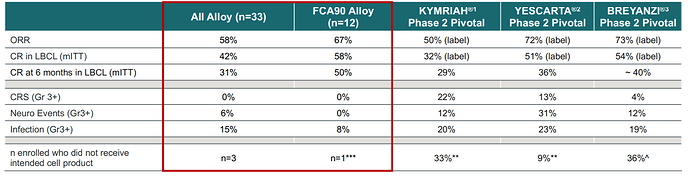
- Initial Clinical Data demonstrated 67% ORR (overall response rates) and 58% CR (Complete response rate) among the 12 patients which is comparable to other Approved CD19 Autologous CAR Ts.
- Safety Profile appears similar or slightly better than Autologous CAR Ts
- ALLO initiated a potentially pivotal Phase 2 study in 3L LBCL for ALLO -501A, complete enrollment by 1h 2024)
- Earlier line Phase 3 readiness expected in 2023; initiation in 1H 2024
- Scalable manufacturing process which allows for treatment within 2-5 days of enrollment
In my opinion the strengths of ALLO-501 and ALLO-501A are the robust durability with 6- and 12-month CR rates of 50%, the “similar” manufacturing process and slightly better safety profile.