I am leveraging multiple sources, so I don’t claim any credit for this.
To make the biopharma/tech plays more accessible I decided to start an education thread on biopharma/tech. Obviously this doesn’t take the risk aware of these plays but hopefully I give you a better understanding of what the play is, the risk associated with it and what we are looking for in terms of result.
Today I would like to start with Common Terms that are used in the Due Diligence of Plays.
Next Topics are:
- How to play biopharma/tech (Data Catalyst, PDUFA, M&A, Patent Catalyst, …)
- How to Interpret Clinical Results
- … (suggestions?)
Clinical Trial
Research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Clinical trials are used to determine whether new biomedical or behavioral interventions are safe, efficacious, and effective. Behavioral clinical trials involving an intervention to modify behavior (diet, physical activity, cognitive therapy, etc.) fit this definition of a clinical trial.
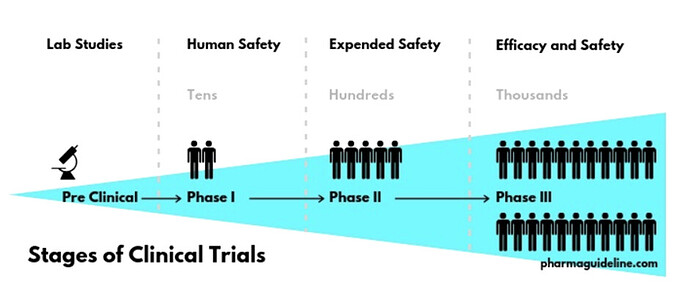
Stages of Clinical Trials
The stage of a clinical trial studying a drug or biological product, based on definitions developed by the U.S. Food and Drug Administration (FDA). The phase is based on the study’s objective, the number of participants, and other characteristics. There are five phases: Early Phase 1 (formerly listed as Phase 0), Phase 1, Phase 2, Phase 3, and Phase 4. Not Applicable is used to describe trials without FDA-defined phases, including trials of devices or behavioral interventions.
Phase 1: A phase of research to describe clinical trials that focus on the safety of a drug. They are usually conducted with healthy volunteers, and the goal is to determine the drug’s most frequent and serious adverse events and, often, how the drug is broken down and excreted by the body. These trials usually involve a small number of participants.
Phase 2: A phase of research to describe clinical trials that gather preliminary data on whether a drug works in people who have a certain condition/disease (that is, the drug’s effectiveness). For example, participants receiving the drug may be compared to similar participants receiving a different treatment, usually an inactive substance (called a placebo) or a different drug. Safety continues to be evaluated, and short-term adverse events are studied.
Phase 3: A phase of research to describe clinical trials that gather more information about a drug’s safety and effectiveness by studying different populations and different dosages and by using the drug in combination with other drugs. These studies typically involve more participants.
Phase 4: A phase of research to describe clinical trials occurring after FDA has approved a drug for marketing. They include postmarket requirement and commitment studies that are required of or agreed to by the study sponsor. These trials gather additional information about a drug’s safety, efficacy, or optimal use.
Endpoints
Endpoints are used in clinical trials to measure exactly how safe and effective a new drug is to help decide whether or not it should be approved. These may measure the survival rate of patients that use the drug, record the frequency of side effects, or identify the best dose to use. At each stage of the clinical trial process, new endpoints will be set that the new treatment must meet before being allowed onto the market.
Overall vs Progression-Free Survival
These are two popular endpoints used during clinical trials, particularly those investigating new cancer drugs. Overall survival measures the percentage of study participants that survive for a set period of time after diagnosis or the initiation of treatment, and boosting this is the best way to prove that a drug is effective. Progression-free survival tracks how long during and after treatment a patient’s disease doesn’t get worse, and it is the next best endpoint for a drug to meet.
Adverse event (AE)
Any untoward or unfavorable medical occurrence in a clinical research study participant, including any abnormal sign (e.g. abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the participants’ involvement in the research, whether or not considered related to participation in the research. Serious Adverse Event (SAE) includes any AD that
- Results in death
- Is life threatening, or places the participant at immediate risk of death from the event as it occurred
- Requires or prolongs hospitalization
- Causes persistent or significant disability or incapacity
- Results in congenital anomalies or birth defects
- Is another condition which investigators judge to represent significant hazards
Arm
A group or subgroup of participants in a clinical trial that receives a specific intervention/treatment, or no intervention, according to the trial’s protocol.
Enrollment
The number of participants in a clinical study. The “estimated” enrollment is the target number of participants that the researchers need for the study.
Placebo
An inactive substance or treatment that looks the same as, and is given in the same way as, an active drug or intervention/treatment being studied.
Efficacy
Indication that the clinical trial intervention produces a desired therapeutic effect on the disease or condition under investigation.
Randomization
The process of assigning clinical trial participants to treatment or control groups using an element of chance to determine the assignments in order to reduce bias.
US Food & Drug Administration (FDA)
As the USA’s equivalent of the EMA, the FDA ensures that new foods, drugs, and cosmetics are safe and effective for use. It also regulates the manufacturing, marketing, and distribution of tobacco to reduce its use by minors. If a particular disease is underserved and patients require new medications as soon as possible, the FDA can also speed up the approval of new drugs by awarding Fast-Track or Orphan Drug Designations.
European Medicines Agency (EMA)
Now that a coin toss has decided that the EMA will move to Amsterdam, it can continue controlling the medicines that make it onto the European market. The agency makes its decisions by looking over the data collected during clinical trials to make sure that new drugs are safe and effective. It is helped by the Committee for Medicinal Products for Human Use (CHMP), which performs the early evaluation of drugs before giving the EMA its opinion. The agency also boosts the development of new medicines and makes sure that patients in need can access them, ensures that drugs on the market are safe post-approval, and provides clear information about medicines to professionals and patients.
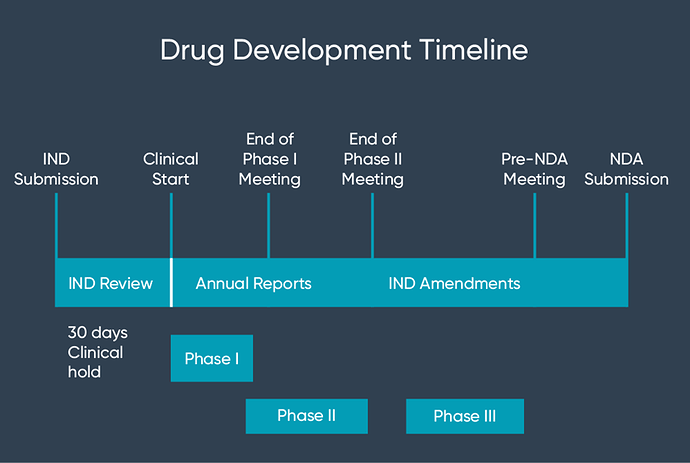
Investigational new drug (IND)
An investigational new drug (IND) application is the first step for any pharmaceutical company on their journey to getting a new drug to market. Submitted to the United States Food and Drug Administration (US FDA), the application is a mandatory requirement to allow clinical investigations on unapproved drugs. Another function of an IND application is to permit shipment of the unapproved drug across state lines, as only marketed drugs are allowed to be shipped from state to state. In which case, the IND acts as a temporary legal function.
There are two forms of INDs:
- Commercial – businesses filing for marketing approval for a new drug
- Research – businesses filing for investigator IND, emergency use IND, and treatment IND, whereby no standard treatment is available and insufficient time exists to receive approval
- The IND application must contain information such as:
- Preclinical data to ensure safety in human testing
- Manufacturing information such as the controls used for producing the drug substance and product
- Clinical protocols to evaluate whether the initial-phase trials pose any unnecessary risks to research subjects
- Investigator information to evaluate the qualifications of the clinical professionals who are overseeing the trials
Once submitted to the FDA, the process will take up to 30 days, after which a drug manufacturer may send the drug to the investigators identified in the application
New drug application (NDA)
After your IND application has been approved and the clinical trials have been completed, a formal request to market the drug must be made in the form of a new drug application (NDA). This application will include all clinical trial data that has been collected through the completed phases carried out following acceptance of the IND.
This is the final step in a pharmaceutical company’s journey to getting their drug to market. Considerably more complex than an IND, this submission will need to encompass a lot more data (15 sections worth) including: pharmacokinetic and pharmacodynamic data, ingredient information, clinical results, and quality control.
- The goal in providing this data is to assure the FDA that:
- The benefits of the new drug outweigh the negatives; safeguarding the effectiveness of the drug
- The intended labeling for the drug is correct and appropriate
- The manufacturing process is suitable to preserve the strength and dosage of the drug
Advisory Committee
An advisory committee lends credibility to the product review process and provides a forum for public discussion of certain controversial issues. The process helps air issues that do not have simple answers. For specific products, advisory committees consider the available evidence and provide scientific and medical advice on safety, effectiveness, and appropriate use. The committee will generally vote on the efficacy and safety of the product. The FDA, while not bound by the committee decision, tend to generally ( but not always ) follow the advice given by the committee when making its decision on or before the PDUFA date. An Advisory Committee meeting is generally convened approximately 2-4 months prior to the PDUFA date.
PDUFA Date & Priority Review
The Prescription Drug User Fee Act (PDUFA) date is the target action date for the FDA to make a decision about a new drug application (NDA). This is sometimes referred by investors as the “FDA Approval date”. The FDA may approve the product or issue a Complete Response Letter (CRL) if they choose not to approve. The CRL will set forth in detail the specific deficiencies and, where appropriate, the actions necessary to place the application in condition for approval.
For NME (new molecular entity) NDA and original BLA submissions, the time period for the FDA to review the NDA is set at 12 months following filing (10 month review following a 60 day filing period). A minority of products are fortunate to receive priority review, where a decision will be issued in 8 months (6 month review following a 60 day filing period). It is common for a decision under priority review to be issued well BEFORE the priority review date.
A Priority Review designation is given to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists.
For a non-NME original NDA the time frame is TEN months following submission and 6 months for a priority review.
The FDA is permitted to extend the PDUFA date under certain circumstances, but is required to inform the the company if this is so:
- A major amendment to an original application, efficacy supplement, or resubmission of any of these applications, submitted at any time during the review cycle, may extend the goal date by 3 months.
- A major amendment may include, for example, a major new clinical safety/efficacy study report; major re-analysis of previously submitted studies or a submission of a REMS (Risk Evaluation and Mitigation Strategies).
- A major amendment to a manufacturing supplement submitted at any time during the review cycle may extend the goal date by two months.