Cingulate (CING) is a biopharmaceutical company focused on the development, manufacturing and commercialization of pharmaceutical products that utilize precision timed release (PTR) drug delivery platform technology to create dosing schedules and drug release profiles that improves the lives of patients suffering from a multitude of commonly diagnosed conditions.¨¨
Pipeline:
The Precision Timed Release (PTR) Platform unlocks the possibility for ‘True’ Once-daily, Multi-dose Tablets. In other words the platform enables the releases of medication at predefined times thus providing the
opportunity for entire day efficacy, safety and convenience. A robust patent portfolio covering the PTR drug delivery platform provides as well strong IP Protection with the opportunity of further developments:
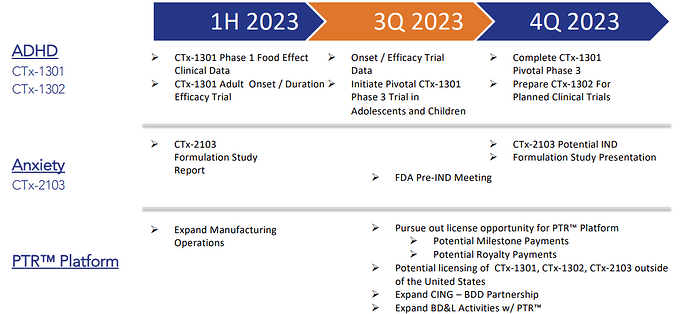
Key upcoming Catalysts:
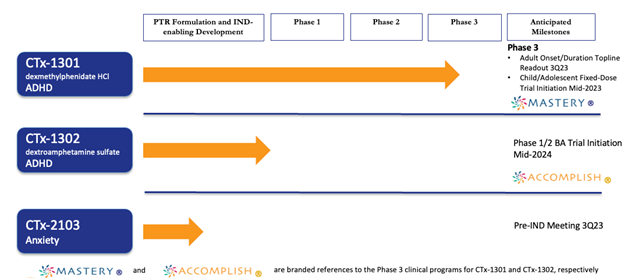
- CTx-1301 (fixed dose, pediatric study) - ADHD - Phase 3: data due in 3Q 2023.
- CTx-1301 (dexmethlyphenidate HCI) - ADHD - Phase 3: initial results expected in 3Q 2023
- CTx-1302 (dextroamphetamine suffate) - ADHD - Phase 1/2: Data expected before early 2025.
Market Opportunity
Total ADHD pharmacotherapy sales in 2022 exceeded $20 billion, with over 90% from the stimulant class. In addition the Anxiety markets is a over $5 billion market in the US with Buspirone as #1 treatment.
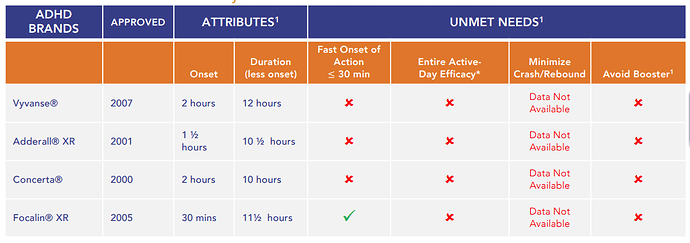
The current major unmet needs of ADHC patients are the need for booster / recovery meds, wear-off effects, abuse of drugs, heavy rebound/crash and lack of fast onset of action:
For example Vyvanse start working within 1.5 hours after taking the medication in a clinical trial of children ages 6 to 12 with ADHD. In a study of adults diagnosed with ADHD, the drug was shown to start working within 2 hours. The rebound effect of Vyvanse can result in an intense reaction or behavior change for roughly 60 minutes at the end of a dose.
So the promises of CTx-1301/CTx-1302, enabled by the The Precision Timed Release (PTR) Platform, is
- Entire active-day duration and fast onset of action
- Elimination of need for a ‘booster/recovery’ dose of short-acting stimulant medication
- Improved tolerability including minimization or elimination of rebound/crash symptoms associated with early medication ‘wear-off’
- Allowing payers to reimburse for one ADHD medication versus two
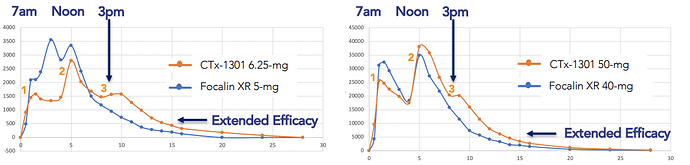
Clinical data demonstrated the following:
- CTx-1301 blood levels demonstrate the potential for a duration of action for the entire active-day, up to 16 hours, vs. Focalin XR 12-hour duration
- CTx-1301 performed as designed, with its precise 20% ’built-in-booster’
- CTx-1301 demonstrates rapid and equivalent blood levels of Focalin XR, indicative of a 30-minute onset of action
- CTx-1301’s precise, 20% 3rd delivery stopped the mid-afternoon plummeting of blood levels, controlling the decline until early evening
- 28.6% reduction in TEAE’s related to CTx-1301 versus Focalin XR (14.3% difference)
Financials:
As of December 31, 2022, Cingulate had $5.4 million in cash and cash equivalents, as compared to $16.5 million in cash and cash equivalents as of December 31, 2021. Based on the Company’s current operating plan, Cingulate expects its cash and cash equivalents will enable the Company to fund its research and development and general and administrative expenditures into the second quarter of 2023. In January 2023, Cingulate entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC, as sales agent (“Wainwright”), pursuant to which we may offer and sell, from time to time through Wainwright, shares of our common stock for aggregate proceeds of up to $2.65 million. To date, Cingulate has not made any sales under the ATM Agreement. In addition, Cingulate is evaluating other alternatives to raise additional capital, including equity and debt financing.
Position: small starter position (announced in the Position System)