Cullinan Oncology (CGEM) is a biopharmaceutical company focused on developing a diversified pipeline of targeted oncology and immuno-oncology therapies with transformative potential for cancer patients. Cullinan’s lead candidate, CLN-081 is an orally available small molecule designed as an irreversible epidermal growth factor receptor (EGFR), inhibitor that is designed to selectively target cells expressing mutant EGFR variants, including EGFR exon 20 insertion (EGFRex20ins), mutations, with relative sparing of cells expressing wild type EGFR.
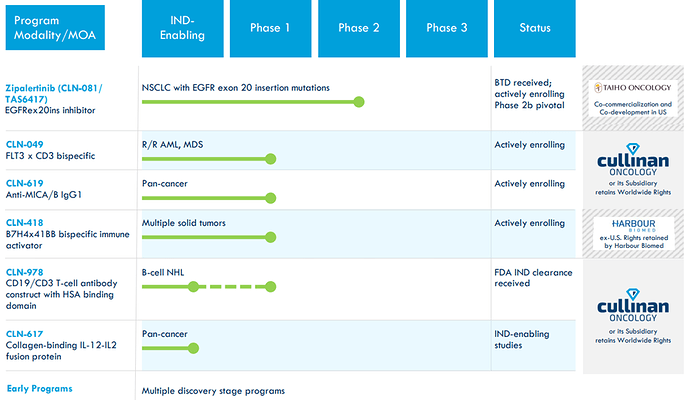
Pipeline:
[size=4]Upcoming Catalysts mid-2023[/size]
Initial Clinical Data from CLN-049 (Phase 1): a FLT3-targeted T-cell engager being investigated in AML.
The only curative therapy for AML is intensive chemotherapy +/- stem cell transplantation, at the same time this therapy remains out of reach for most AML patients because 85% patients are over 60 years old are ineligible for intensive chemotherapy. So, there is a significant unmet need remains for a broadly applicable therapy that can produce high rates of durable response.
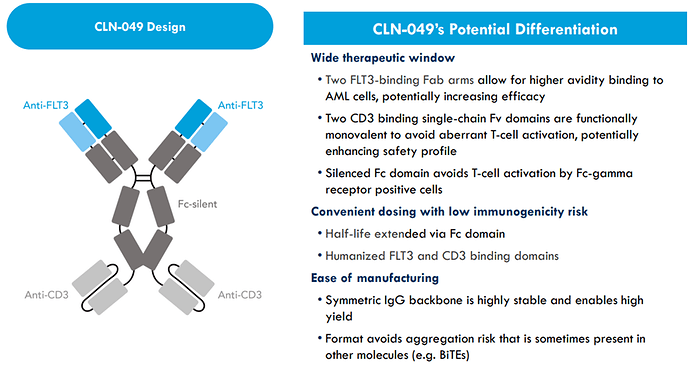
CLN-049 is a novel T-cell engager targeting FLT3:
Preclinical data supports mechanism of FLT3-dependent T-cell activation and broad AML cell elimination resulting in improved survival:
Initial Clinical Data from CLN-619 (Phase 1): prevent immune evasion through MICA/B shedding and has pan-cancer potential.
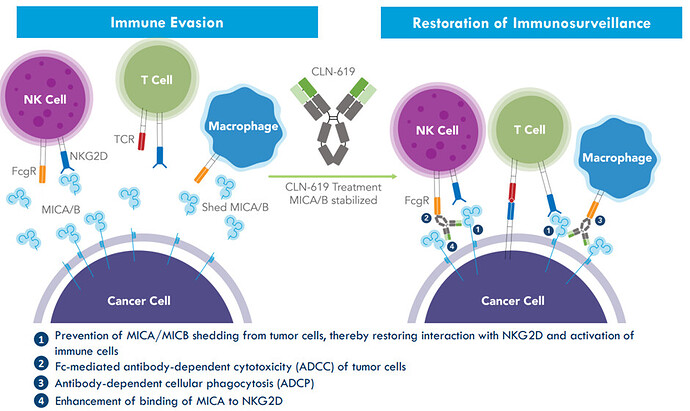
MICA/B serves as a warning signal to the immune system to eliminate potentially dangerous cells:
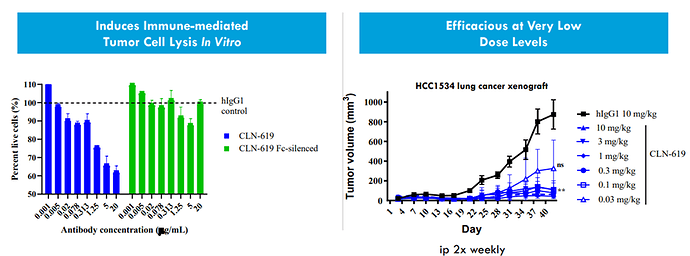
CLN-619 is seen as first-in class potential as it’s the only antibody which is at the clinical stage, and it does not require preconditioning with immune ablation. The Strong pre-clinical data supporting and the Multiple modes of action underlines the first-in class potential:
[size=4]Key Catalysts (2024)[/size]
Lead program: zipalertinib (CLN-081/TAS6417) for NSCLC EGFR exon 20 initiated a pivotal study 4Q 2022, Data in 2024
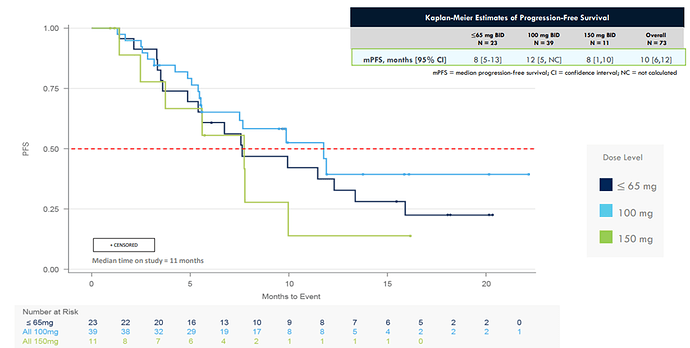
CLN-081 demonstrated in an ongoing Phase 1 / 2a Study 41% (remember this number) confirmed overall rate of response (ORR) and 12-month (and this number) median progression-free survival (PFS). Compared (see table below) to real-world data from other 1st line treatment outcomes in exon20 NSCLC patients this is means CLN-081 could be a best-in-class drug:
| Overall rate of response (ORR) | Median progression-free survival (PFS), months | |
|---|---|---|
| Any Therapies | 18.6% | 5.2 |
| Platinum Based Chemotherapy | 19.5% | 5.7 |
| Checkpoint Inhibitor plus Platinum Based Chemotherapy | 18.8% | 4.5 |
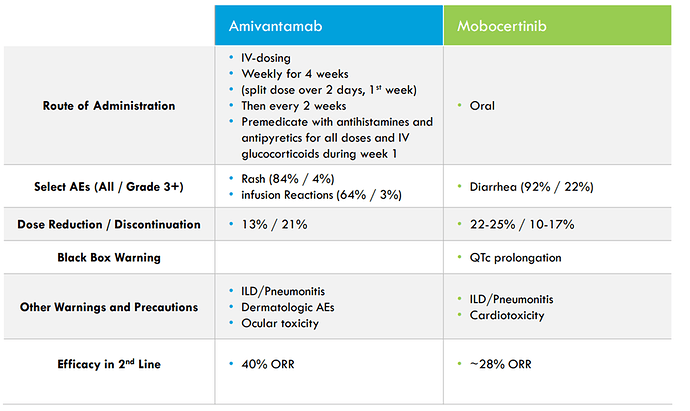
Compared to approved 2nd line therapies which have meaningful benefit, but safety/tolerability and durability leave a large unmet need:
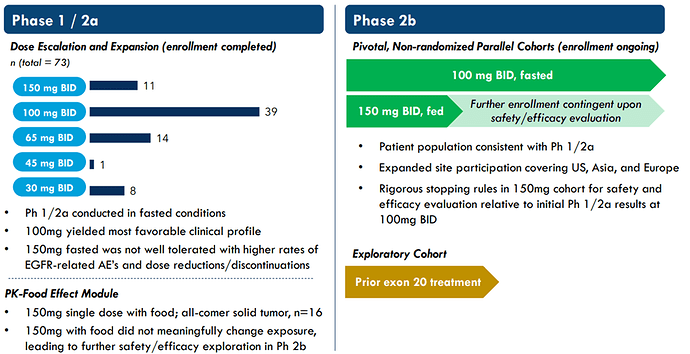
Hence the FDA granted Breakthrough Therapy Designation for CLN-081 in January 2022. The Phase 1 / 2a is still ongoing and will be expanded, at the company start to enroll a phase 2b study to expand the scale and focus on the 100mg dosing:
The start of the Pivotal Phase 2b study, the Breakthrough Therapy Designation and the focus on the 100mg BID, which demonstrated the best PFS (see table below) and safety profile is a indication to me that the company, and investors/partners. believe in the potential of the drug.
CGEM entered a partnership as well with Taiho to maximizes shareholder and patient value, commercialization potential, and investment. Taiho Oncology is focused on small molecule, molecularly targeted therapeutics and actively advancing 7 molecules in approximately 20 solid-tumor clinical trials. Most important Taiho commercialized three oncology products, LONSURF, INQOVI, and LYTGOBI (futabatinib, FGFR1-4 inhibitor), so they have a comprehensive U.S. oncology commercial infrastructure in place, including salesforce, marketing, market access, and medical affairs capabilities. As a result of the partnership there will be a US 50/50 profit share and upfront payments.
initial clinical data from CLN-418: a B7H4 x 41BB bispecific immune activator with potential to address multiple solid tumors.
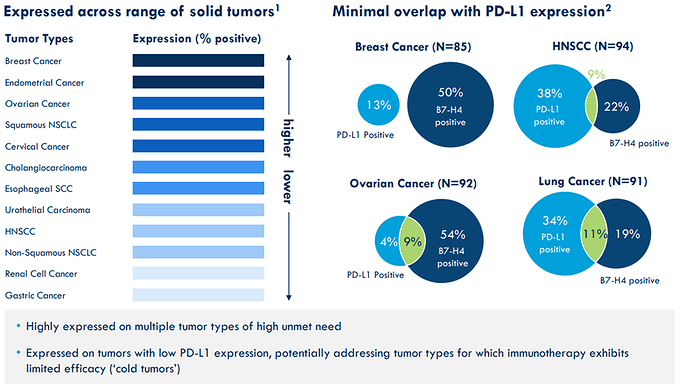
B7H4 protein is widely expressed across multiple tumors with low expression on normal tissue. It has a minimal overlap with PD-L1 (Remarks by President Biden on the Economy: “We finally beat Pharma” – Did we? - #2 by Genti) treatments which creates opportunity where existing IO approaches have limited efficacy. So B7H4 is an attractive target for cancer immunotherapy:
CLN-418 has the potential to first in class treatment for cancer patients where PD-L1 treatments had limited efficiency and patients have a high exposure of the B7H4 protein.
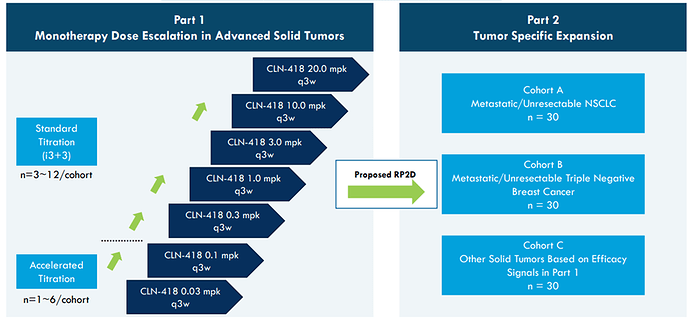
The CLN-418 Study is a first-in-human study to evaluate the safety and tolerability of the study drug CLN-418, and to determine the maximum tolerated dose and/or recommended Phase 2 study dose of CLN-418:
Phase 1 study underway focusing on monotherapy dose escalation, combination development planned. Initial clinical data expected 2024.
Financials
Robust cash position of $535m (runway through 2026) allows them to prosecute clinical programs more efficiently, including CLN-619 parallel dose escalation as monotherapy and pembrolizumab combination.
Position: will start a significant long-term position, only play this if you are interested in the long-term and don’t need the capital.