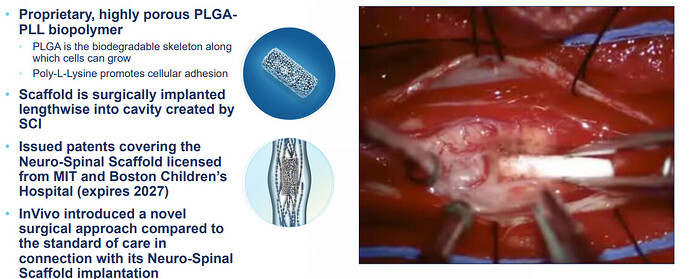
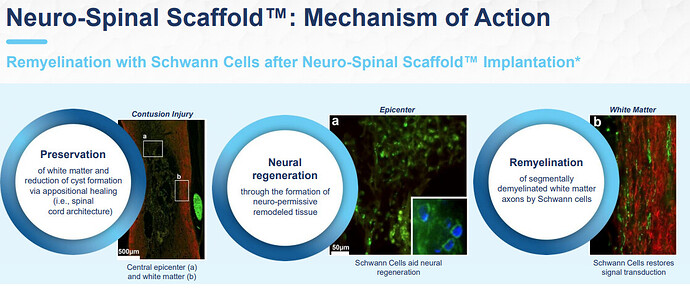
InVivo Therapeutics (NVIV) is a research and clinical-stage biomaterials and biotechnology company focused on the treatment of spinal cord injuries (SCIs). The Company’s Neuro-Spinal Scaffold implant is an investigational bioresorbable polymer scaffold that is designed for implantation at the site of injury within a spinal cord. NVIV does not have any commercialized products and upcoming catalyst is basically their full pipline.
Spinal Cord Injury (SCI)
Despite significant advances in surgical repair to the spinal column over recent decades, modern day acute management of SCI does not address repair of the spinal cord. The Standard of care (SOC) is an early decompressive surgery that attempts to remove ongoing .spinal cord compression.
The Unmet Medical Need:
- Approximately 17,000 new cases of acute SCI per year in US
- Patients affected by loss of motor, sensory and autonomic (bowel, bladder and sexual) function
- Only small percentage of patients ever regain function3
- Approximately 285,000 currently live with chronic SCI in US
- Cost of care for the first year post-SCI: $350K - $1.0M+
- $4.8M+ Net present value of the cost for a quadriplegic injured at 25 for life
The goal is to establish the Neuro-Spinal Scaffold as the foundation of the standard of care for acute SCI.
NVIV investigational Neuro-Spinal Scaffold
Clinical and Regulatory Development Pathway so far
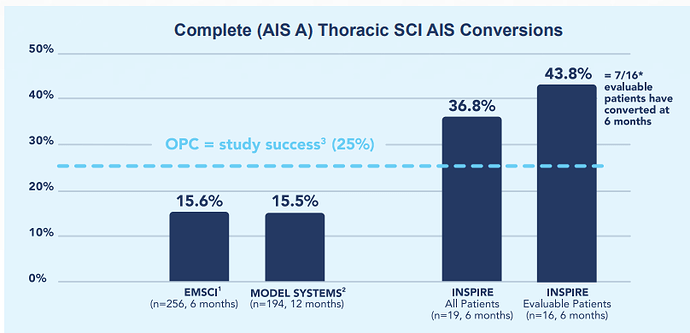
The investigational NeuroSpinal Scaffold was implanted in a total of 19 patients in the INSPIRE 1.0 Study, 16 (84%) of whom reached the six-month primary endpoint visit. Three patients died within 3 weeks of implantation (all of which were deemed unrelated to the investigational device or surgical procedure by the respective site)
Upcoming Catalyst: Top-line data expected in Q1-2023 for INSPIRE 2.0

Financials
$10.6M in cash and cash equivalents at September 30, 2022; no debt. Estimated that these cash resources will fund company operations into the first quarter of 2024
Position: Shares (announced in the position system)