Disclaimer: High Risk / High Reward because Krystal Biotech currently has no commercialized product, so zero revenue. The current evaluation of the company is 100% based on the potential pipeline, B-VEC is the only near term catalyst.
- Krystal Biotech (KRYS) is a biotechnology company focused on developing and commercializing genetic medicines for patients with rare diseases. The Company’s wide-ranging pipeline is based on its proprietary redosable HSV vector. vector optimization, gene therapy manufacturing and commercialization.
- BLA (Biologics License Applications) for B-VEC (GEM-3) accepted August 18, 2022. PDUFA Priority Review date February 17, 2023
- B-VEC is an investigational treatment for dystrophic epidermolysis bullosa (DEB), a rare disease that renders patients’ skin extremely fragile and susceptible to blisters even for otherwise ordinary things such as scratching an itch.
- The standards of care for DEB focus on treating those wounds. B-VEC would help address the disease at a deeper level.
- Krystal Biotech estimates that there are roughly 9,000 DEB patients in the markets it will target, including 3,000 in the U.Sd
- New England Journal of Medicine Publishes Phase 3 Data on B-VEC in Patients with Dystrophic Epidermolysis Bullosa
- In this GEM-3 trial of 31 patients, complete wound healing at 6 months occurred in 67.4% of B-VEC wounds compared to 21.6% for placebo (difference, 45.8 percentage points; 95% confidence interval [CI], 23.6 to 68.0; p=0.002). Complete wound healing at 3 months occurred in 70.6% of the wounds exposed to B-VEC as compared with 19.7% of those exposed to placebo (difference, 51.0 percentage points; 95% CI, 29.3 to 72.6; p=0.0005).
- The GEM-3 trial was a randomized, double-blind, intra-patient placebo-controlled multi-center trial designed to evaluate the efficacy and safety of B-VEC for the treatment of DEB. In the trial, matched wounds receiving topical B-VEC or placebo were evaluated in 31 DEB patients over 26 weeks. The pivotal GEM-3 trial met its primary endpoint of complete wound healing at six-months and its secondary endpoint of complete wound healing at three-months.
- B-VEC was well tolerated, with no drug-related serious adverse events or discontinuations due to treatment.
About B-VEC
B-VEC is an investigational non-invasive, topical, redosable gene therapy designed to deliver two copies of the COL7A1 gene when applied directly to DEB wounds. B-VEC was designed to treat DEB at the molecular level by providing the patient’s skin cells the template to make normal COL7 protein, thereby addressing the fundamental disease-causing mechanism.
The FDA and EMA have each granted B-VEC orphan drug designation for the treatment of DEB, and the FDA has granted B-VEC fast track designation and rare pediatric designation for the treatment of DEB. In addition, the FDA granted Regenerative Medicine Advanced Therapy (RMAT) to B-VEC for the treatment of DEB and the EMA granted PRIority MEdicines (PRIME) eligibility for B-VEC to treat DEB.
About Dystrophic Epidermolysis Bullosa (DEB) (dont google it for you are eating currently)
DEB is a rare and severe disease that affects the skin and mucosal tissues. It is caused by one or more mutations in a gene called COL7A1, which is responsible for the production of the protein type VII collagen (COL7) that forms anchoring fibrils that bind the dermis (inner layer of the skin) to the epidermis (outer layer of the skin). The lack of functional anchoring fibrils in DEB patients leads to extremely fragile skin that blisters and tears from minor friction or trauma. DEB patients suffer from open wounds, which leads to skin infections, fibrosis which can cause fusion of fingers and toes, and ultimately an increased risk of developing an aggressive form of squamous cell carcinoma which, in severe cases, can be fatal.
More information on: Dystrophic epidermolysis bullosa: MedlinePlus Genetics
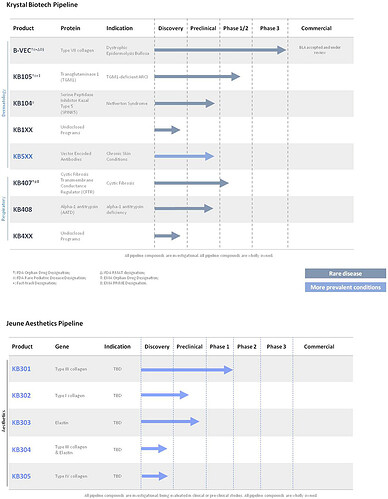
Pipeline and other catalysts:
- KB104 - Netheron Syndrome: IND (Investigational New Drug) filing anticipated in 2022.
- KB407 - (CORAL-1) - Cystic Fibrosis: IND cleared August 1, 2022 with trial initiation expected in 4Q 2022.
- KB301 - (PEARL-1)- Wrinkles and acne scars: Phase 1 initial efficacy data reported that subject satisfaction scores had a 21.9% responder rate difference between KB301 and placebo and mean change in skin thickness was 1.07mm between KB301 and placebo, noted March 22, 2022. Durability trial data demonstrated up to nine-month durability of effect following administration of high-dose treatment, noted November 17, 2022. Phase 2 trial planned for 1H 2023.
- KB105 - Transglutaminase-1 Deficient Autosomal Recessive Congenital Ichthyosis (ARCI): Phase 2 update announced July 1, 2021 - well tolerated with no adverse events. Phase 1/2 dosing to initiate early 2023.
Position: no position yet