Viking Therapeutics (VKTX)
- Viking Therapeutics (VKTX) is a biopharmaceutical company focused on the development of therapies for metabolic and endocrine disorders.
- Lead clinical program’s drug candidate, VK2809, is an orally available, tissue and receptor-subtype selective agonist of the thyroid hormone receptor beta
Upcoming Catalysts:
- VK0214 - X-linked adrenoleukodystrophy (X-ALD): Phase 1b trial initiation announced June 23, 2021. Phase 1b trial clinical hold lifted July 19, 2022. Phase 1b results due in 1H 2023.
- VK2809 - (VOYAGE) - Non-alcoholic steatohepatitis (NASH) and fibrosis: Phase 2b enrollment to be complete in 4Q 2022 with data expected 1H 2023.
- VK2735 - Metabolic disorders, healthy volunteers: Phase 1 trial initiated January 10, 2022. Phase 1 data due in early 2023.
Madrigal Pharmaceuticals (MDGL)
- Madrigal Pharmaceuticals (MDGL) is a clinical-stage biopharmaceutical company focused on the development and commercialization of therapeutic candidates for the treatment of cardiovascular, metabolic, and liver diseases.
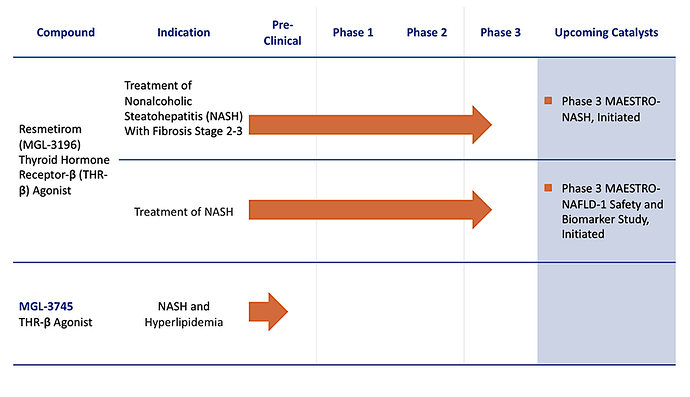
- Lead product candidate, resmetirom, is a liver-directed, selective thyroid hormone receptor-ß (THR-ß) agonist developed as a once-daily oral pill to treat a number of disease states, including non-alcoholic steatohepatitis (NASH).
Upcoming Catalysts:
- Resmetirom (MGL-3196) - (MAESTRO-NASH) - Non-alcoholic steatohepatitis (NASH): Phase 3 biopsy data due in 4Q 2022. NDA filing anticipated in 1H 2023.
As is highlighted in the tile, the focus is on the Non-alcoholic steatohepatitis (NASH) treatment:
- VKTX: Phase 2b data expected 1H 2023.
- MDGL: Resmetirom (MGL-3196): Phase 3 biopsy data due in 4Q 2022. NDA filing anticipated in 1H 2023.
So MDGL will read out their data of Phase 3 before VKTX read out of Phase 2b. I believe they are heavily correlated. For MDGL it will be a binary catalyst.
Position: VKTX MAY23 4C as Sympathy Play and gives me a 2nd chance their data is better if MDGL fails. MDGL has the higher Risk but also higher reward.
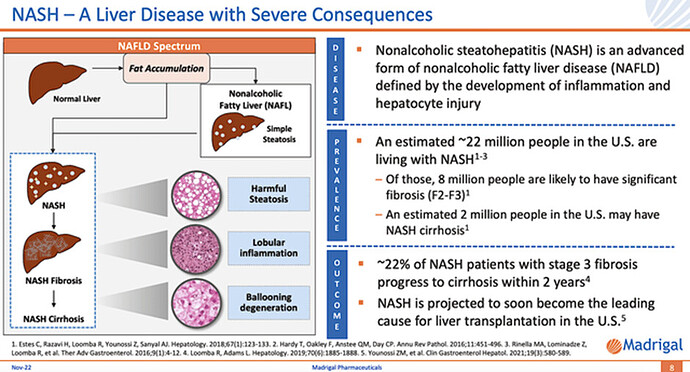
Disease Context: Non-alcoholic Steatohepatitis
In the earlier stage, fatty liver accumulation is the dominant characteristic of NASH. For the later phase, the diseased liver would experience NASH fibrosis with fibrotic (i.e., scar) tissue buildup. And, the degree of fibrosis is graded from F2 to F4 level. In the last stage, the NASH liver becomes cirrhotic and thereby loses its function.
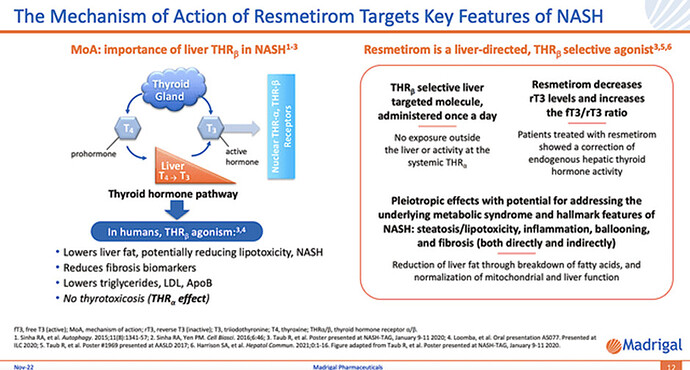
Mechanism of Action (MIA) for Resmetirom
By removing the excess fat with Resme, the liver’s innate healing capability kicks in to shift the balance toward regeneration. As you can appreciate, the DC and MOA fit together like matching puzzle pieces that would indicate positive clinical future data.