Thank you @Fllwoman for bringing ORMP to my attention.
Summary: ORMP has the potential to create a new paradigm in the treatment of type 2 diabetes by orally delivering insulin, but the need to raise cash – assuming positive data result – to effort submission and commercialization is a risk in this play.
Oramed Pharmaceuticals (ORMP) is a pharmaceutical company It is engaged in the research and development of pharmaceutical solutions, including an oral insulin capsule to be used for the treatment of individuals with diabetes, and the use of orally ingestible capsules or pills for delivery of other polypeptides.
The true value of this company is breakthrough platform technology for oral delivery of drugs and vaccines presently available only via injection:
The pipeline currently focused on diabetes, with potential for a variety of additional treatment indications addressing multi-billion-dollar markets. Type 1 Diabetes projected Market by 2029 is $24b, Type 2 is 92b
Key Catalyst: Results from Oramed Pharmaceuticals’s first phase 3 study ORA-D-013-1, using ORMD-0801 for the treatment of patients with Type 2 Diabetes, is expected January 2023.
ORA-D-013-1, the first of our two pivotal Phase 3 oral insulin trials for ORMD-0801 in the treatment of type 2 diabetes met and exceeded the number of planned participants, with 710 people enrolled. We expect to announce topline results in the next four weeks.
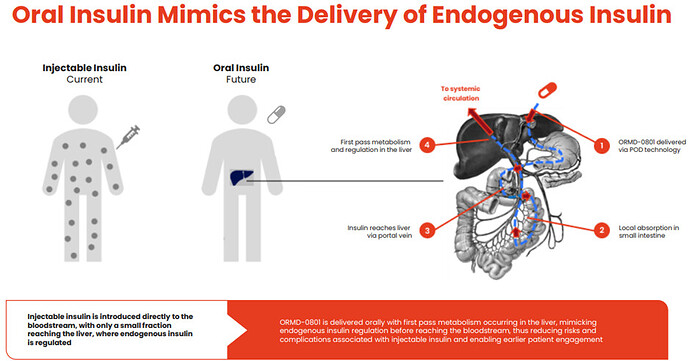
Highly simplified ORMP offers oral insulin instead of injectable insulin for Type 2 Diabetes Patients. For type 1 Diabetes the clinical research in still in phase 2a. I believe ORMD-0801 has the potential to create a new paradigm in the treatment of type 2 diabetes by orally delivering insulin even at an earlier stage of treatment.
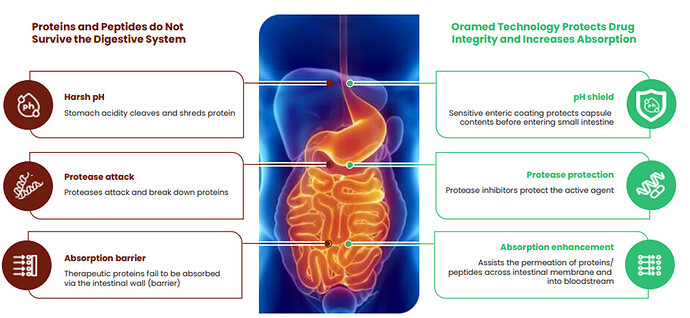
The company sees the following significant Advantages Over Injectable Insulins
- Improved Blood Glucose Control: Insulin is regulated endogenously in the liver, limiting the amount of excess systemic insulin that can lead to hypo/ hyper-glycemic events
- No weight Gain: Better insulin control prevents cells from absorbing excess glucose that can be converted to fat and lead to weight gain
- Ease of Administration: Oral delivery benefits diabetic patients with a fear of needles and should improve patient administration and compliance
- Earlier treatment should slow disease progression and delay late-stage complications
Oramed successfully completed a Phase 2 and Phase 2b Trial:
- Achieved primary endpoint and showed significant positive effects
- Safe/well-tolerated with no drug related serious adverse events
- All ORMD-0801 values statistically significant versus the placebo (p-Value<0.05)
- ORMD-0801 showed promising reductions in mean 24-hour, fasting, and daytime glucose levels
- All ORMD-0801 values statistically significant versus the placebo (p-Value<0.05)
- Achieved primary efficacy endpoint in reduction in A1C at Week 12
- The 8 mg once-daily and twice-daily arms achieved statistically significant values at Week 12 vs. Placebo (p-value 0.028 and 0.029, respectivel
- ORMD-0801 upheld safety profile previously exhibited in first Phase 2 study (No increase in Serious Adverse Events compared to Placebo, No increase in Hypoglycemic Events compared to Placebo, No weight gain compared to Placebo at Week 12)
As a Catalyst we are looking at the results of the following Trial:
Risks
- The most significant risk is obviously the actual data results, even if previously evidence confirms positive sentiment and the company seems quite confident about the upcoming data read outs.
- From a financial point of view ORMP sits currently on ~ $160M (as of 09/30/22) Cash. Oramed believes that it has enough cash to fund its operations for at least the next 12 months, but I believe they will – and need to - raise cash combined with positive data results to effort the BLA submission and the commercialization of the product. The Commercial Agreement with Medicox (South Korea) and the related milestone payments/royalties on future gross sales demonstrates the need for cash.
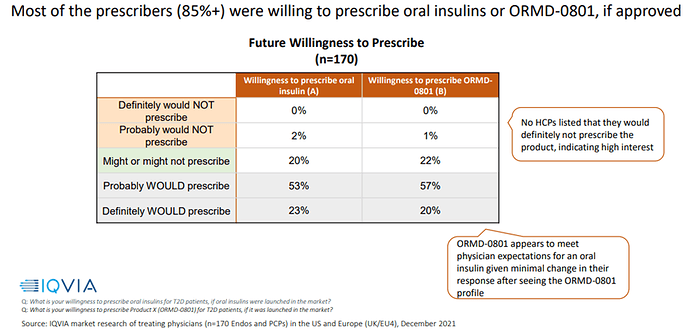
- One other risk I see if Healthcare Professional (HCPs = Doctors) are willing to prescribe oral insulins given their resilience to change and the potential of “making less money”. ORMD shared the following research on this topic which is in my eyes overconfident:
Other Cataclyst: ORMD-0801 - Oral Virus-like Particle (VLP) - COVID-19 vaccine: Phase 1 complete results due in 1Q 2023. Phase 1 trial first patient enrolled December 14, 2021.
Our subsidiary Oravax’s first cohort in its Phase 1 clinical trial of our oral virus-like particle (VLP) COVID-19 vaccine for COVID-naive participants achieved its primary and secondary endpoints of safety and immunogenicity based on preliminary data. Our oral vaccine elicited a potentially protective IgG response with significant seroconversion from baseline. We believe this trial shows proof of concept for oral delivery of vaccines using Oravax’s technology. Complete Phase 1 results are expected in the first quarter of 2023.
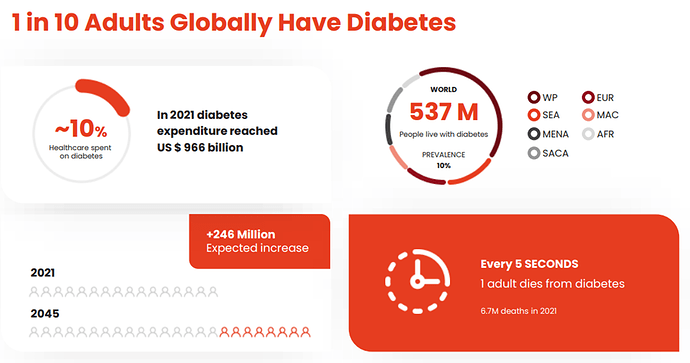
About Diabetes
Diabetes is a disease in which your blood glucose, or blood sugar, levels are too high. Glucose comes from the foods you eat. Insulin is a hormone that helps the glucose get into your cells to give them energy. With type 1 diabetes, your body does not make insulin. With type 2 diabetes, the more common type, your body does not make or use insulin well. Without enough insulin, the glucose stays in your blood. You can also have prediabetes. This means that your blood sugar is higher than normal but not high enough to be called diabetes. Having prediabetes puts you at a higher risk of getting type 2 diabetes.
Over time, having too much glucose in your blood can cause serious problems. It can damage your eyes, kidneys, and nerves. Diabetes can also cause heart diease, stroke and even the need to remove a limb. Pregnant women can also get diabetes, called gestational diabetes.
Read more on https://medlineplus.gov/diabetes.html
Position: ORMP FEB23 15C & Shares