hey guys, first time poster here. i wanted to write up a starter DD to get this on people’s radar since the approval is coming up quickly and hopefully others can chime in and add to this discussion. this has been on my watchlist for a while and my main play on this ticker is the PDUFA approval set for Tuesday, Nov 30, 2021.
WHO ARE THEY
VBI Vaccines, Inc (VBIV) is a $723 million market cap commercial-stage biopharmaceutical company that is developing products to target infectious diseases. for this DD we’ll be focused on their products that target Hepatitis B and to a lesser degree, Coronavirus.
WHAT’S THE PLAY and WHY DO WE CARE
the main play is their 3-antigen prophylactic HBV vaccine candidate set for PDUFA approval next Tuesday, Nov 30th, 2021. this product targets Hepatitis B virus which affects roughly a shit-ton of people worldwide.
“Hepatitis B is one of the world’s most significant infectious disease threats with more than 290 million people infected globally. HBV infection is the leading cause of liver disease and, with current treatments, it is very difficult to cure, with many patients going on to develop liver cancers. An estimated 780,000 people die each year from complications of chronic HBV such as liver decompensation and hepatocellular carcinoma.”
but wait, aren’t there Hepatitis B vaccines already available? why yes, yes there is. the current vaccines are 1-antigen and VBIV has the only 3-antigen product. to my layman’s intellect, 3 is better than 1. don’t take my word for it, read the below double-blind studies published within the past year regarding their product. if you don’t want to read them, the conclusion: that VBIV’s tri-antigenic hepatitis B vaccine (TAV) safety and efficacy performed better than the mono-antigenic vaccine (MAV).
https://www.businesswire.com/news/home/20210202005180/en/VBI-Vaccines-Announces-U.S.-FDA-Acceptance-of-BLA-Filing-for-VBI’s-3-Antigen-Prophylactic-Hepatitis-B-Vaccine
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30780-5/fulltext
https://www.jhep-reports.eu/article/S2589-5559(21)00137-3/fulltext
COMPANY FINANCIALS
Based on their 3rd quarter earnings report:
VBI ended the third quarter of 2021 with $137.5 million in cash, cash equivalents, and short-term investments compared with $93.8 million as of December 31, 2020.
Net cash used in operating activities for the nine months ended September 30, 2021 was $21.4 million, compared to $30.6 million for the same period in 2020. The decrease in cash outflows is largely a result of the change in operating working capital, notably the cash received in advance from the CEPI funding agreement, offset by an increase in net loss.
with biopharm stocks, the fear of dilution is always on the table. they have plenty of cash so my personal opinion is that dilution isn’t a short-term threat to the share price.
PIPELINE
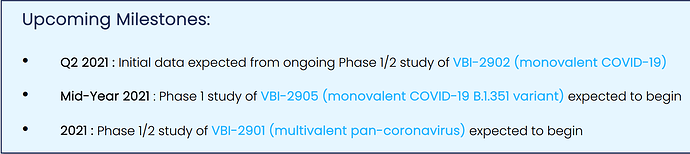
i mentioned that my main play is the PDUFA approval for their Hepatitis B vaccine but they also have several other products coming down the pipeline. they’ve been active in the Coronavirus vaccine space.
they’ve partnered with several companies to produce covid vaccines. they have multiple covid vaccines in the pipeline.
“There are 4 separate vaccines in development. And whilst none have yet made it past the Phase 1 study stage, they have been designed, according to management, to complement existing vaccines, as boosters or to provide additional durability.”
they’re behind the covid vaccination race but if you they can deliver, it could be a substantial revenue base for them in 2022. this is why this could be a mid/long term hold besides the short-term PDUFA play.
CHARTS and TA
charts and technical analysis aren’t a factor since this is an approval play. if anyone wants to add their take on the charts, by all means, please do so.
TLDR
their Hepatitis B vaccine is up for PDUFA on Nov 30th. their share price is near the yearly lows and sitting at $2.74 as of this writing. i believe if approved, this should pop quite a bit.
i’ve played several biopharm plays on this server: ALLO, AVDL, EYEN, OYST to name a few. many of those didn’t live up to the hype of holding through approval BUT you would have made profits (many did) just by playing the runup to the approval. the IV for VBIV’s Dec options are fairly low right now, around 100.
there are several ways to play this:
- play the run up and the IV increase
- play for the approval and hold through the approval date
- buy shares and play long term for their Hepatitis B and Coronavirus vaccines to play out
any critique or suggestions are welcomed.
thanks.